
 Virus removal filtration, as one of the robust ways of virus removal, is widely used in the production of biopharmaceuticals. Based on size exclusion, virus removal filters can remove all kinds of viruses in a robust way, and can conduct post process integrity test to ensure the effectiveness of virus retention, which has been widely accepted by the industry.
Virus removal filtration, as one of the robust ways of virus removal, is widely used in the production of biopharmaceuticals. Based on size exclusion, virus removal filters can remove all kinds of viruses in a robust way, and can conduct post process integrity test to ensure the effectiveness of virus retention, which has been widely accepted by the industry.
The most common materials for virus removal filters include polyether sulfone (PES) and regenerated cellulose (RC). Among them, PES membrane is recognized by high flux, high capacity and high retention, it has good performance in antibody products. However, for high concentration or relatively unstable proteins, like plasma or fusion protein, PES is easy to plug and the flux may decrease sharply as the filtration time increase due to its high sensitivity. In which case, RC membrane filter has good performance in the production of plasma products, recombinant protein and other biological products due to its superior hydrophilicity.
The challenge of virus removal in filtration step of plasma products
Plasma products refer to various human plasma protein products separated and extracted from human plasma. Plasma contains water, saccharides, electrolytes, and other protein components, of which protein only accounts for 7%. Plasma products are different components obtained through multi-step separation and purification, including human albumin, immunoglobulin, coagulation factor and other protein components. Among them, intravenous immunoglobulin (IVIG) has a wide field of application, and there is a large space to expand the indications. At present, the consumption of IVIG in APAC market is far lower than the international level, so there exists a high demanding on the market.
After years of process development, the concentration of IVIG on the market is mainly 5% (50g/L), some preparations can reach as high as 10% or even 20%. IVIG generally uses saccharides as stabilizer, and there is a certain proportion of dimer. For virus removal filtration step, high concentration and complex composition will lead to high viscosity of feed solution, low flow rate and easy to block. Cost has become the bottleneck of virus removal filter to be widely used in plasma production process. Increase of capacity and reduce of cost is the key of virus removal filter.
S&P virus removal filtration solution for plasma product
Valpha? RC HP is the new generation of virus removal filters made of regenerated cellulose membrane by S&P filtration, which provides robust virus safety guarantee for plasma products such as high concentration IVIG. Based on the structural improvement of regenerated cellulose, RC HP has a stronger capacity to tolerant impurity. It can provide virus removal filtration process of plasma products a stable flux, leading the process to achieve the target of robust virus retention and high flux with low cost.
1. Robust virus security
PP7 bacteriophage is a virus with bacteria as host. It structures in icosahedron, and sizes from 23 to 26 nm. The PDA TR 41 has mentioned that PP7 bacteriophage could be used as the model virus for filter manufacturer using in releasing test. S&P used PP7 bacteriophage to conduct retention test on 15 batches of membranes of Valpha? RC HP. The test results are shown in the following figure, and the LRV is higher than 6.
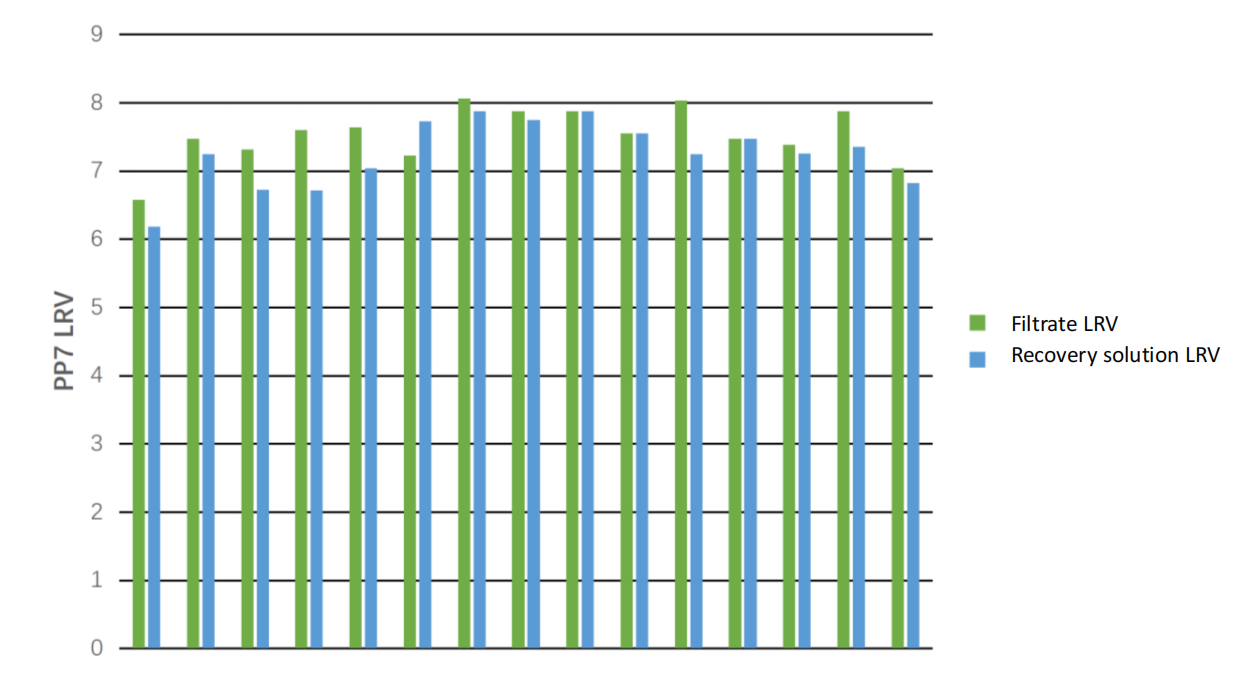
Figure 1 Valpha? RC HP PP7 bacteriophage retention capacity
2. Robust performance on high concentration IVIG
Case 1
Sample: 5% IVIG after two-step chromatography
Purity: 98%
pH:4.10
Conductivity: 2.96 ms/cm
Challenge virus: 2% PPV (porcine parvovirus)
Test pressure: 3 bar
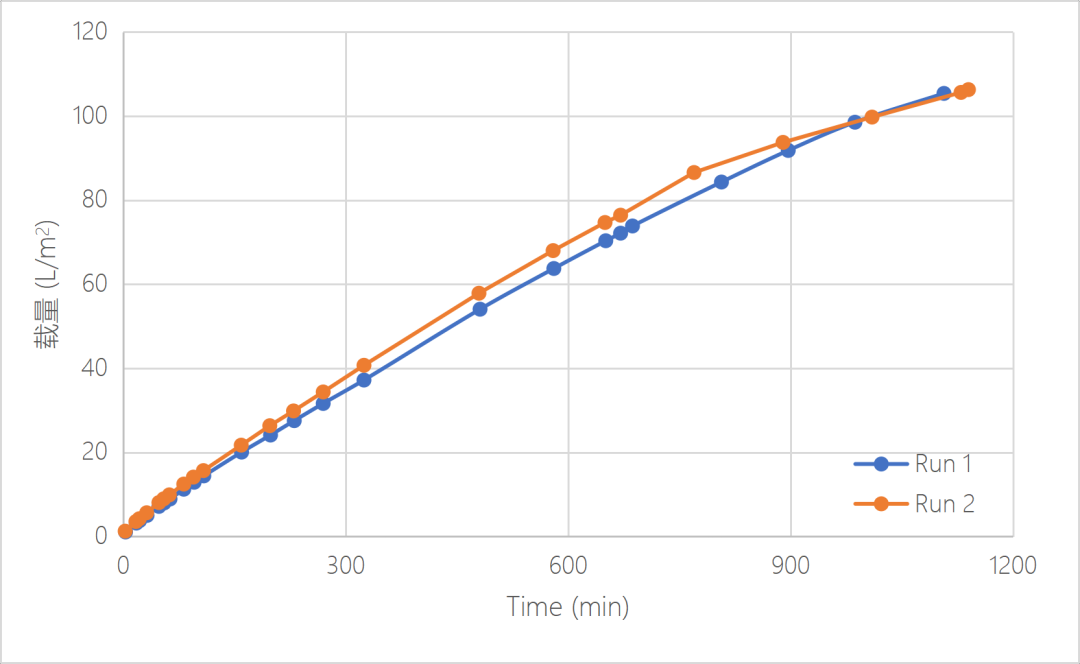
When filtrate high concentration IVIG with virus, Valpha? RC HP can still maintain a stable flux, the flux attenuation is less than 50%, the volume capacity can reach>106L/m2, and the mass capacity can reach > 5.3kg/m2. After 18 hours filtration, no virus particle was detected in the permeate solution and the recovery solution, and the final LRV>4.
Case 2
Sample: 5.2% IVIG after anion flow chromatograph
Purity: 99%
pH:3.63
Conductivity: 1.25 ms/cm
Challenge virus: 2% PPV (porcine parvovirus)
Test pressure: 3 bar
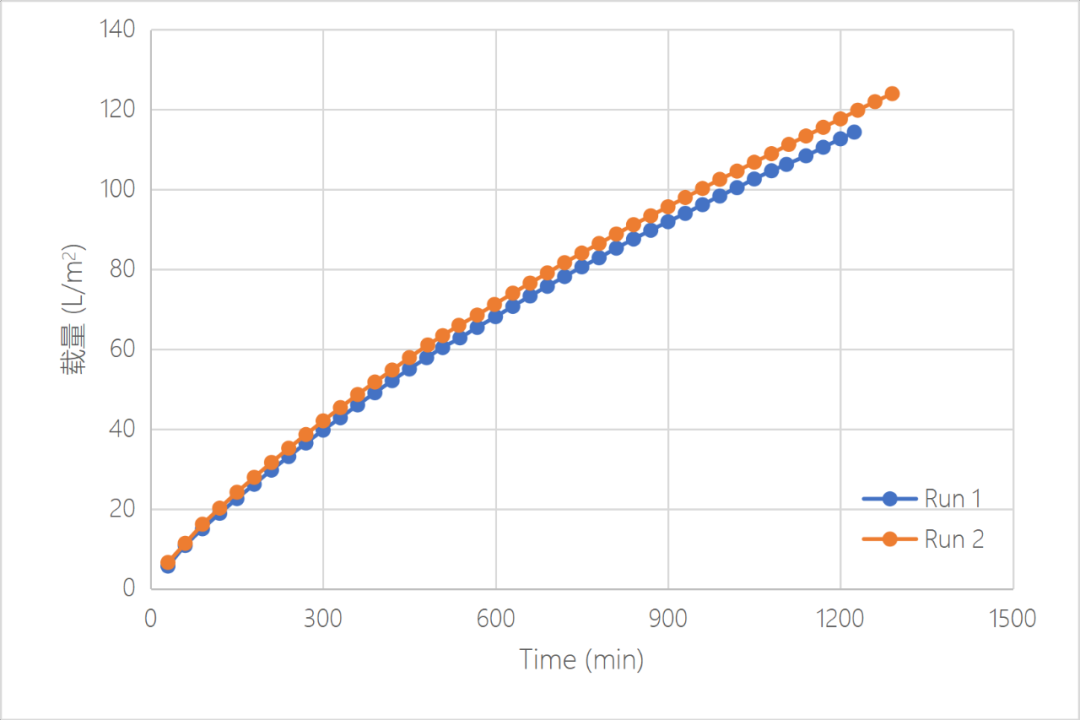 When filtrate high concentration IVIG with virus, Valpha? RC HP can still maintain a stable flux, the flux attenuation is less than 60%, the volume capacity can reach>124L/m2, and the mass capacity can reach > 6.4kg/m2. After 21.5 hours filtration, no virus particle was detected in the permeate solution and the recovery solution, and the final LRV>4.
When filtrate high concentration IVIG with virus, Valpha? RC HP can still maintain a stable flux, the flux attenuation is less than 60%, the volume capacity can reach>124L/m2, and the mass capacity can reach > 6.4kg/m2. After 21.5 hours filtration, no virus particle was detected in the permeate solution and the recovery solution, and the final LRV>4.
Ending
Valpha?RC HP can provide stable filtration process for high concentration IVIG. Even when the virus is added, the capacity can reach 5-7kg/m2, which can greatly reduce the process cost of plasma products.
Valpha?RC HP can provide powerful virus security guarantee, which can effectively retentate parvovirus in long time filtration process and ensure LRV>4.
Thank you for your time reading, If you have any product related technical questions, please feel free to contact your local sales representative or through Email:market@www.cbsantnarcis.com
SAIPU (Hangzhou) Filtration Technology Co.,Ltd